
That means SO 3 has 24 valence electrons. Sulfur brings 6, and oxygen brings 3 each. Valence: Here, sulfur in the center because of its lowest electron capability, and three oxygen around it. (By the way, that is the reason why SO 3 is having the shape of Trigonal Planar.) The bond angle of SO 3 is 120 degrees. That means we have AX3 for the SO 3 molecule. Moreover, as there are three oxygen, it will be X3. In this formula of SO 3, we don’t have any non-bonding electron, and that is why we don’t bother about N. N stands for any nonbonding electron pairs.A stands for Sulfur, which is central atom For Example, Sulfur Dioxide, So2, Electron-domain Geometry Is Trigonal Planar.These electrons are negative and repel each other. There are one sulfur atom and three oxygen atoms which are spread out as far away as they can! Atoms of oxygen are surrounded by electrons. SO 3 includes two components mainly – Sulfur and Oxygen.

It can determine reactivity, polarity, color, attraction, biological activity, etc.

Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule. SO3 Hybridization Sulfur Trioxide Molecular Geometryīeing an intelligent and well-practiced human being, you must know what is molecular geometry, but let me revise it for the all young students out there.If there are one or more lone pairs on the central atom, the molecular geometry (the actual shape of the molecule) will not be the same as the electron domain geometry. For example, two electron domains gives a linear molecule three domains gives a trigonal planar molecule four domains gives a tetrahedral structure five dom. If there are no lone pairs around the central atom, refer to the table below (Table below), to determine the molecular geometry, which is the same as the electron domain geometry. Electron geometry refers to the geometry of electron domains around the central atom and can be determined from a formula (Niles, 2004). Shape is determined by the number of both shared and unshared domains of electrons around the central atom and refers only to the orientation of atoms in and around the center.Ĥ. The molecular geometry can be determined from the Lewis Dot diagrams because it is determined by the number of electron domains around the central atom (Niles, 2004). The Lewis Dot structure is used to represent the valence electrons in chemical bonding, and the Lewis Dot structures are useful for explaining the chemical bonding in ions and molecules (Chieh, 2004). The molecules are considered isoelectronic because they both have three hydrogen atoms attached to a highly negative atom and have the same electronic potential.ģ. The nitrogen atom has a lone pair of electrons so it acts as a base. The NH3 molecule also has a trigonal pyramidal configuration, giving the molecule an overall dipole moment and making it polar (Ammonia, 2005). The hydronium ion, H3O, has a positive charge on one of the H ions in the molecule, which has a trigonal pyramidal configuration. The molecule is trigonal planar because there are three electron domains around the central sulfur atom.Ģ.
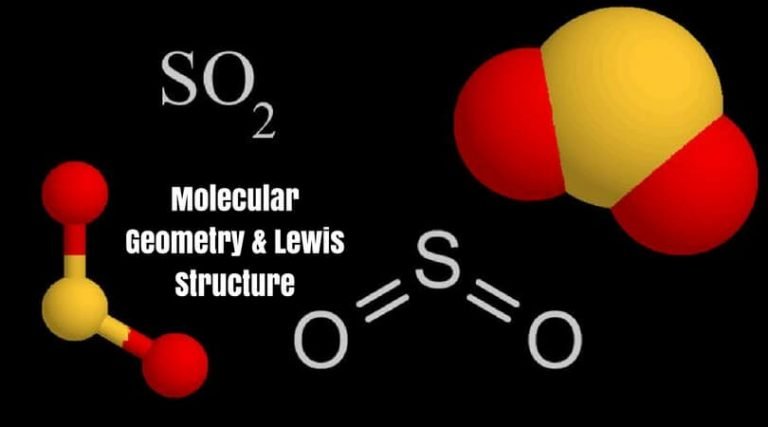
The molecule has an S-O single bond and an S=O double bond. The SO2 molecule is a bent molecule, existing as a resonance structure, with a lone pair of electrons on the S. There is no charge on the molecule because there are no lone electron pairs. There are two electron domains around the central carbon atom, therefore it is linear (Niles, 2004). The difference in polarity between CO2 and SO2 can be explained by their molecular shape.


 0 kommentar(er)
0 kommentar(er)
